teachers'domain: Digital Media for the Classroom and professional Development
User: Preview
Media Type:
Video
Running Time: 2m 49s
Size: 16.4 MB
Astronomical Images in Different Wavelengths
(Interactive)
Making Waves with the Electromagnetic Spectrum
(Lesson Plan)
Tour the Electromagnetic Spectrum
(Interactive)
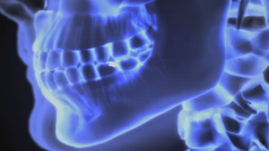
Viewing X-Rays
(Interactive)
National K -12 Subject:
WGBH is trying to develop materials that better meet the needs of our users. Please take this brief survey to share how you use these resources and to provide feedback on your experiences using these materials. Take the Survey!
Electromagnetic Spectrum
The electromagnetic – or EM – spectrum is made up of seven kinds of electromagnetic energy with each corresponding to a different range. From lowest energy to highest energy, the seven groupings along the spectrum are: Radio Waves, Microwaves, Infrared, Visible Light, Ultraviolet, X-rays, and Gamma Rays. Electromagnetic energy travels in waves and spans a broad spectrum from very long radio waves to very short gamma rays. Read in the order listed above, waves increase in frequency and decrease in wavelength. The visible range, which is the only portion of the spectrum the human eye can detect, represents only a very tiny portion of the entire electromagnetic spectrum.
All electromagnetic radiation is made up of up of fields of electricity and magnetism interacting with each other. Electric fields can be static – like the static electricity that can hold a balloon to the wall. Magnetic fields can be static, too – like what holds a refrigerator magnet in place. However, electric and magnetic fields can also change and move together, and when that happens, the interaction produces waves: electromagnetic waves. EM energy can be described by frequency, wavelength, or energy, all of which are inter-related by the expression E = Frequency / Wavelength. Frequency is directly proportional to energy (they increase and decrease together) while wavelength is inversely proportional to energy (as wavelength increases, energy decreases).
Radio and microwaves are usually described by frequency (units of Hertz), infrared and visible light by wavelength (units of meters), and x-rays and gamma rays by energy (units of electron-volts). Though referred to by different names – light, EM radiation, or rays – all EM energy is made up of the same kinds of waves. The convention of using different units for different parts of the spectrum is simply a convenience that has to do with using numbers that are neither too large nor too small. The distinctions between the energy bands are simply a convention that eases communication. The EM spectrum doesn’t actually have breaks or chapters.
When you think of a water wave in the ocean, it might be easy to imagine the water oscillating up and down, creating a traveling waveform across the water. Even easier to imagine: making waves travel along a jump-rope secured to a wall at one end. In that case, it’s easy to see that the wave’s oscillation is perpendicular to the direction of its forward movement. In other words, the movement of the rope may be up and down, but the wave that travels through the rope is moving forward or backward—two perpendicular directions. These kinds of waves are called “transverse” waves. In transverse waves, the direction of the wave is perpendicular to the direction of applied energy. Another type of wave is a “longitudinal” wave, in which the wave moves parallel to the applied energy. With sound waves and other longitudinal waves, molecules vibrate and bump into one another, passing energy along the same direction the wave is moving.
While some transverse waves and some longitudinal waves might be easy to imagine, electromagnetic wave are harder to visualize. Because the wave is traveling in a direction that’s perpendicular to both the electric field and the magnetic field, thinking about EM waves requires three-dimensional visualization. Transverse waves like the jump-rope example give a close approximation, but electromagnetic radiation is more complex. One important feature of EM radiation is that, since its movement is based on the interaction of electric and magnetic fields, and electric and magnetic forces are possible over long distances, EM waves can travel through a vacuum. No material medium is necessary. Remember that low-energy EM radiation has longer wavelengths, corresponding to lower frequencies. High-energy EM radiation has shorter wavelengths, corresponding to higher frequencies.
All EM radiation travels at the same speed: the speed of light.
The categories along the spectrum – Radio, Microwave, Infrared, Visible, Ultraviolet, X-ray, Gamma Ray - represent a useful breakdown of EM radiation that helps scientists understand and visualize energy sources on, in, and under the Earth as well as throughout our solar system, galaxy, and universe.
X-rays
X-rays have very small wavelengths, 3 nm to .03 nm, about the size of an atom. This means they have very high frequencies and thus very high energies. At this region of the EM spectrum, where light is so energetic, it is able to penetrate deeper and thus becomes potentially more harmful to living cells. Our atmosphere screens out most harmful EM radiation, including x-rays, although some UV rays do pass through.
X-ray telescopes have to be launched into orbit to do their viewing from above the atmosphere. Astronomers often compare views of the same areas of space in different wavelengths – x-ray and infrared, for example. Through such comparisons across the EM spectrum, scientists get more complete views of sources of EM radiation.
Astronomers use x-ray sensors to capture images and information about everything from the Sun, to pulsars, supernovae, black holes, and other sources. The events and objects that emit x-rays are very hot and very energetic. X-ray telescopes capture data and images that help scientists figure out the composition, temperature, density, and movement of the observed objects – including those from the earliest moments of the universe.
Before
During
After
NARRATION: A star explodes in a blinding supernova, spraying X-rays across the galaxy to tell its tale. X-rays also tell a dentist which tooth to drill, and a surgeon which bones to mend. In 1895, Wilhelm Röntgen discovered that firing streams of X-rays through arms and hands created eerie, but detailed, images of the bones inside.
X-rays are high energy light rays with wavelengths between 3 and 0.03 nanometers, so small that some X-rays are no bigger than many individual atoms. In laboratories, scientists fire beams of X-rays at unknown substances to learn what elements they contain and to decode their atomic structure. This is how scientists unraveled complex molecules like penicillin and DNA.
Scientists can also detect the X-rays emitted from extremely hot and energetic objects in the universe. NASA’s robotic rovers recorded X-rays to identify the spectral signatures of elements, such as zinc and nickel, in Martian rocks.
X-rays can also reveal an object’s temperature, since temperature determines the wavelength of its radiation. The hotter the object, the shorter that wavelength is. X-rays come from objects that seed at millions of degrees, such as pulsars, black holes, supernovas, or the plasma in our Sun’s corona. Our Sun has a surface temperature of around 6 thousand degrees Celsius and radiates most of its energy in visible wavelengths. But it is easier to study the massive energy flows in the corona’s energetic plasma by observing X-rays, like this image from the Hinode satellite, a joint Japanese-NASA mission. NASA’s SOHO satellite produced these X-ray images of the Sun that allows scientists to see and record these energy flows within the corona.
NASA’s orbiting Chandra X-ray observatory detects X-rays created by objects spread far across space, such as this supernova explosion that occurred 10 thousand light years from Earth. The colors of the gas and dust cloud correspond to different energy levels of the X-rays created by the blast.
X-rays at different wavelengths provide information about an object’s composition, temperature, density, or its magnetic field. Human eyes may not be able to see X-rays, but, from seeding cosmic bodies to individual atomic elements, X-rays provide a wealth of information to exploring scientists.
To help improve this service, please report and describe any standards correlations that you find to be inaccurate.
Academic standards correlations on Teachers' Domain use the Achievement Standards Network (ASN) database of state and national standards, provided to NSDL projects courtesy of JES & Co.
![]()
We assign reference terms to each statement within a standards document and to each media resource, and correlations are based upon matches of these terms for a given grade band. If a particular standards document of interest to you is not displayed yet, it most likely has not yet been processed by ASN or by Teachers' Domain. We will be adding social studies and arts correlations over the coming year, and also will be increasing the specificity of alignment.
You must be signed in to see standards matches for your state.
 Loading Standards
Loading StandardsMajor funding for Teachers' Domain was provided by the National Science Foundation.
Teachers Domain® Home | Change Edition
About Teachers' Domain | Contact Us | Privacy Policy | Terms of Use
Teachers' Domain: © 2002-2025 WGBH Educational Foundation | shopPBS Educational Media




