Teachers' Domain - Digital Media for the Classroom and Professional Development
User: Preview
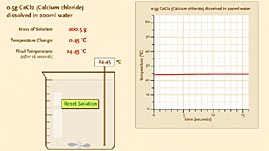
Source: Thomas J. Greenbowe and the Iowa State University Chemical Educational Group
In this interactive activity adapted from Iowa State University, investigate how salts dissolve in water. Dissolve different ionic compounds in water, and experiment with varying the amounts of solute and solvent. Use a graph to see and compare how different salts produce exothermic or endothermic changes in enthalpy, which produces changes in temperature.
A solution forms when one substance, the solute, is dissolved in another substance, the solvent. In their interaction, the solute breaks down into smaller particles that are completely surrounded by particles of the solvent. For example, table salt, sodium chloride (NaCl)—a lattice of sodium and chloride ions joined by ionic bonds—dissolves in water by dissociating into Na+ and Cl- ions that are then surrounded by water molecules. This can be written as: NaCl(s) --> Na+(aq) + Cl-(aq).
All salts are ionic compounds, made of positive and negative ions. The process of dissolving a salt in water involves three main steps: breaking the ionic bonds of the salt, breaking the hydrogen bonds in the water, and bonding water molecules to the ions (this process is called hydration). As with any chemical reaction where bonds are broken or formed, energy (generally in the form of heat) is involved. The measure of this energy, or heat content, is referred to as enthalpy. As a solute dissolves, the "heat of solution"—the change in enthalpy—can be measured as a change in the temperature of the solution. If the process of dissolving absorbs heat, the temperature of the solution decreases and the heat of solution is described as endothermic. On the other hand, if the process of dissolving releases heat, the temperature of the solution increases and the heat of solution is described as exothermic.
The hot and cold packs that are often found in first-aid kits make use of different heats of solution. Two components are inside a hot or cold pack: an ionic compound and a pouch containing water. When someone strikes the pack, the inner pouch of water breaks open. The compound dissolves as the water and ionic compound interact. Dissolving different ionic compounds produces different heats of solution. For example, calcium chloride (CaCl2) is used in hot packs because it produces an exothermic heat of solution. Conversely, ammonium nitrate (NH4NO3) is used in cold packs because it produces an endothermic heat of solution.
Even though some bonds are broken and others are formed as the compounds dissolve, this reaction is not generally considered to be a chemical change, which is usually defined as the production of a new substance. In this case, since no new substance is produced, the process of dissolving is usually considered a physical change. For example, when NaCl is dissolved in water, the sodium and chloride ions are separated from one another. However, if the water were to be removed (by evaporation, for example), the ions would simply rejoin and once again form NaCl.
 Loading Standards
Loading Standards Teachers' Domain is proud to be a Pathways portal to the National Science Digital Library.
Teachers' Domain is proud to be a Pathways portal to the National Science Digital Library.
