Teachers' Domain - Digital Media for the Classroom and Professional Development
User: Preview
Source: University of Alberta and Bio-DiTRL
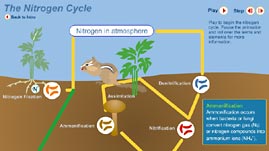
This interactive activity adapted from the University of Alberta illustrates how, through a process called fixation, nitrogen flows from the atmosphere, into the soil, through various organisms, and back to the atmosphere in a continuous cycle.
Nitrogen is the most common element in Earth's atmosphere, yet it is also one of the most limiting factors for growth in plants and animals. Most nitrogen exists as a gas. In this common molecule, two nitrogen atoms are bound by a strong triple bond that makes them all but completely unavailable to any other atom, ion, compound, or organism. Yet without sufficient levels of available nitrogen, organisms would be unable to create their structures or to perform vital functions. Nitrogen is a key building block in a number of important molecules, such as nucleic acids, amino acids, and proteins. Without it, life as we know it would be impossible.
Two natural forces are responsible for most of the gaseous nitrogen that is "fixed," or made available to, plants and animals. Electricity in the form of lightning is one such force. When a bolt of lightning travels through the atmosphere it breaks the triple bond holding the nitrogen gas molecule together, enabling free nitrogen atoms to bond with oxygen in the air to form nitrogen oxides. These compounds dissolve in atmospheric moisture to form nitrates that then fall as rain.
The other force, although less spectacular than lightning, is no less energetic. Countless bacteria, including those living freely in the soil and those found on the roots of some types of plants, fix more than ten times the nitrogen released by lightning strikes. These single-celled organisms break the bonds in nitrogen gas molecules and combine free nitrogen atoms with hydrogen to form ammonium, an ion that is readily absorbed by plants. Some types of nitrogen-fixing bacteria have formed symbiotic relationships with certain types of plants. Legumes, such as peas and beans, support colonies of bacteria called rhizobium, in special structures called nodules, which appear directly on their roots. While the bacteria provide the plants with nitrogen, the plants provide the bacteria with energy-rich carbohydrates and a moist environment in which they can thrive.
Human activities have severely altered the nitrogen levels in some ecosystems. Although nitrogen is typically a limited resource in many environments, it has been made available in massive quantities as a result of agricultural and industrial practices. Sometimes described as "nutrient pollution," the result has often been catastrophic. For instance, when waste such as nitrogen-rich sewage and fertilizers pours into ponds, lakes, and streams, the result is an overabundance of algae (also know as an algal bloom). The eventual death of these microscopic plants leads to their decomposition by bacteria—a process that uses vast quantities of oxygen. Following an algal bloom, decomposing bacteria in lakes and ponds become so abundant and oxygen depletion so complete that fish and other aquatic life may die. In the years since scientists discovered this connection, cities and farmers have taken measures to control the flow of nitrogen into ecosystems, so that this powerful element is available at the right quantity where it is needed and less pervasive where it is not.
 Loading Standards
Loading Standards Teachers' Domain is proud to be a Pathways portal to the National Science Digital Library.
Teachers' Domain is proud to be a Pathways portal to the National Science Digital Library.
