Teachers' Domain - Digital Media for the Classroom and Professional Development
User: Preview
Source: PhET, Physics Education Techonolgy, University of Colorado
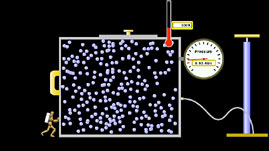
In this interactive simulation adapted from University of Colorado’s Physics Education Technology project, explore the relationship between the pressure, volume, and temperature of a gas. Begin by observing a molecular model in which gas molecules move freely, occasionally colliding with one another and with the walls of their container. By varying the parameters in the model—such as volume, temperature, pressure, number of molecules, and gravity—you can observe changes to the gas system and examine correlations among those parameters.
All matter is composed of atoms and molecules, or particles. The movement and interaction of these particles determine the three main phases of matter: solid, liquid, and gas. In a solid, the particles jiggle in place, but they are not able to move around. Therefore, a solid has a fixed volume (the amount of three-dimensional space it occupies) and shape. In a liquid, the particles are free to slide past each other but remain in close contact. Because of this, a liquid has a fixed volume but can adapt its shape to the container that it occupies. In a gas, the particles move freely, allowing it to assume both the shape and volume of its container—and making the gas compressible and expandable.
A gas has three measurable properties: pressure (P, the force exerted by the gas over a given area), temperature (T, the average energy of motion of the particles), and volume (V). Proportionality is expressed by the symbol ∝. The relationship between these properties is described by fundamental gas laws:
For a sample of gas with constant volume, pressure is directly proportional to temperature: As temperature increases, pressure also increases; as temperature decreases, pressure also decreases. In other words, if volume is constant, P ∝ T.
Similarly, for a sample of gas with constant pressure, volume is directly proportional to temperature: As temperature increases, volume also increases; as temperature decreases, volume also decreases. Thus, if pressure is constant, V ∝ T.
However, for a sample of gas with constant temperature, volume and pressure are inversely proportional: As volume increases, pressure decreases; as volume decreases, pressure increases. In other words, if temperature is constant, P ∝ 1/V.
The behavior of gases can be demonstrated in the kitchen. For example, when making popcorn, the water vapor inside the corn kernel increases in temperature while the volume of the kernel stays the same. The pressure builds inside the confined space of the kernel until finally the kernel bursts open with a pop. Alternatively, when baking bread, the pockets of gas in the dough (formed by fermenting yeast during the rising process) increase in temperature while the pressure stays the same. The hot oven increases the temperature, causing the pockets of gas to expand in volume and the dough to transform into a larger loaf.
 Loading Standards
Loading Standards Teachers' Domain is proud to be a Pathways portal to the National Science Digital Library.
Teachers' Domain is proud to be a Pathways portal to the National Science Digital Library.
